C. Scott Hartley, Ruiyao Wang, and Robert P. Lemieux*
Chem. Mater. 2004, 16, 5297–5303
[Published version]
Abstract
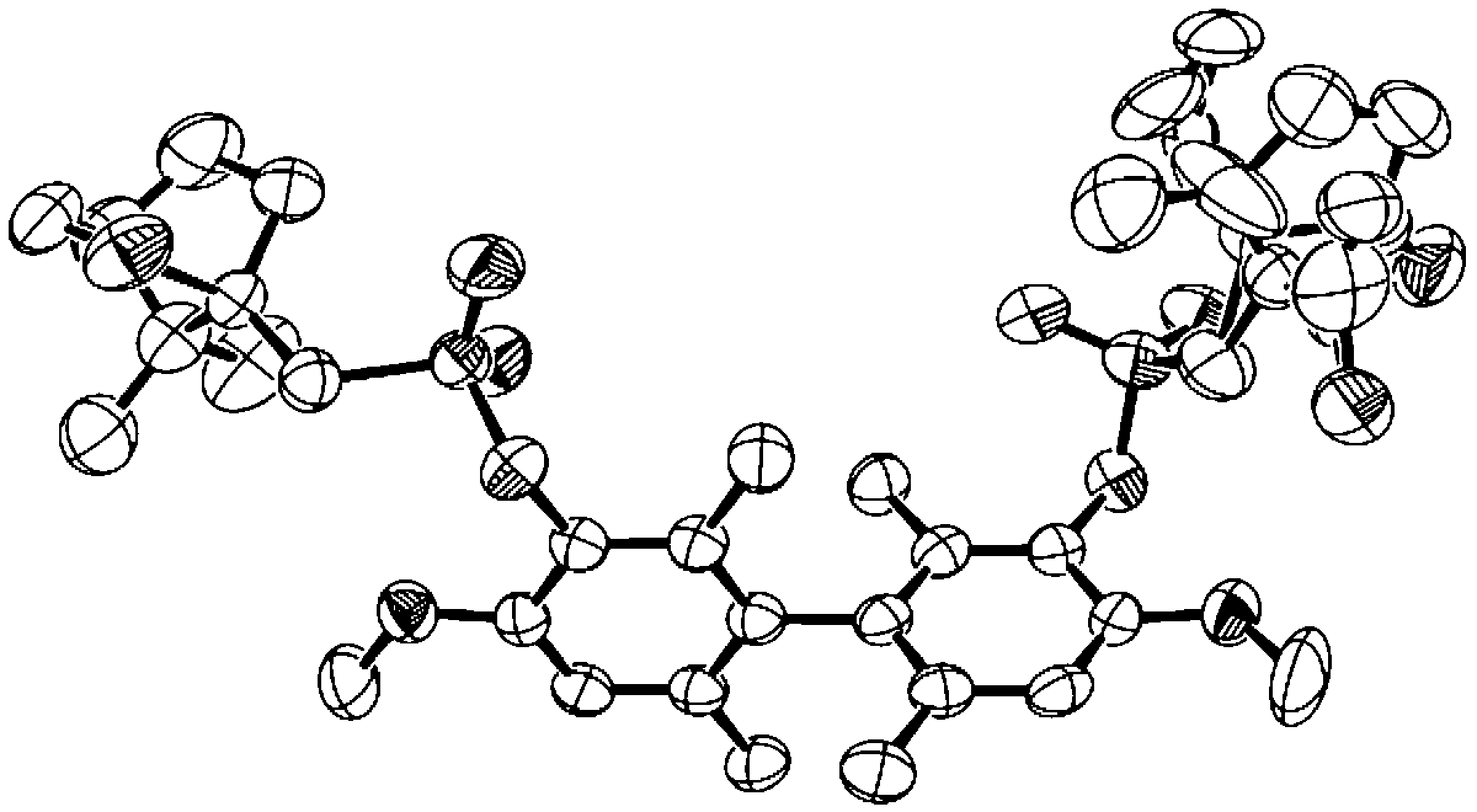
A new synthetic route to a known series of five chiral dopants with atropisomeric biphenyl cores was developed which enabled the assignment of their absolute configurations. These compounds can be doped in achiral SmC liquid crystal hosts to give ferroelectric SmC* liquid crystals with a spontaneous polarization PS that scales with the dopant concentration. One of these dopants, (R)-2,2′,6,6′-tetramethyl-3,3′-dinitro-4,4′-bis[(4-nonyloxybenzoyl)oxy]biphenyl ((R)-1), exhibits one of the highest polarization powers ever reported. The atropisomeric cores were obtained in optically pure form via the resolution of bis-(1S)-10-camphorsulfonamido diastereomers, which were then hydrolyzed to the corresponding diaminobiphenyl enantiomers (X = NH2), and converted to the substituted biphenyl cores (X = NO2, F, Cl, Br, Me) using standard functional group interconversion methods. The absolute configurations of the five chiral dopants were assigned based on the X-ray crystallographic analysis of the diastereomer (R)-3,3′-bis[(1S)-10-camphorsulfonamido]-2,2′,6,6′-tetramethyl-4,4′-dimethoxybiphenyl, and correlated to the sign of spontaneous polarization each dopant induces in the achiral SmC host 2-(4-butyloxyphenyl)-5-octyloxypyrimidine (PhP1).
