Govinda Prasad Devkota, William P. Carson, and C. Scott Hartley*
J. Org. Chem. 2023, 88, 1331–1338
[Published version | NSF-PAR | Preprint | Raw data]
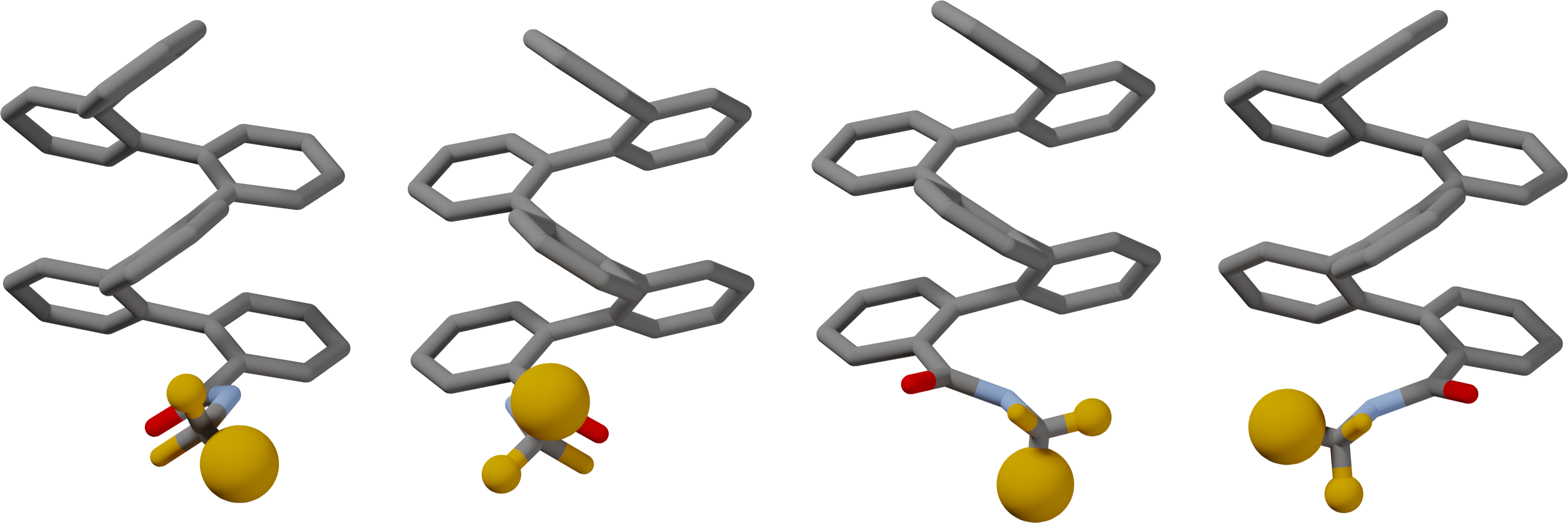
Abstract
Control over the folding of oligomers, be it broad induction of a preferred helical handedness or subtle changes in the orientations of individual functional groups, is important for applications ranging from molecular recognition to long-range conformational communication. Here, we report a series of ortho-phenylene hexamers functionalized with achiral and chiral amides at their termini. NMR spectroscopy, taking advantage of F-19 labeling, allows multiple conformers to be detected for each compound. In combination with CD spectroscopy and DFT calculations, specific geometries corresponding to each conformer have been identified and quantified. General conclusions about the effect of sterics and the amide linker on conformational behavior have been drawn, revealing some similarities and key differences with previously reported imines. A model for twist sense control has been developed that is supported by computational models.
