Yinhui Liao, Govinda Prasad Devkota, Vipul Batra, Jacob R. Franklin, Sean B. Ginn, Diamond D. E. Browner-Smith, David L. Tierney, and C. Scott Hartley*
J. Org. Chem. 2025, 90, 16025–16031
[Published version | NSF-PAR | Preprint | Raw data]
Abstract
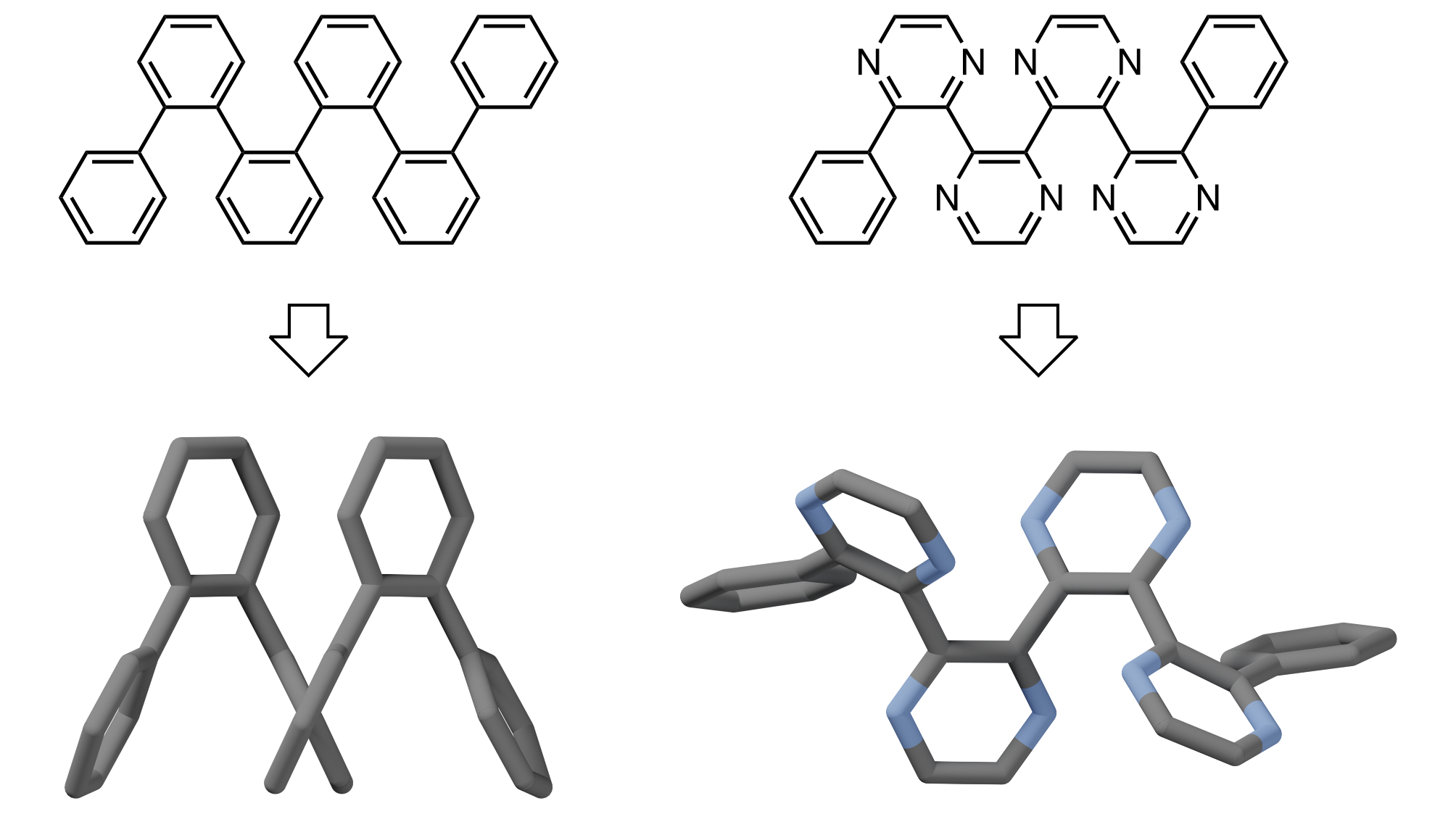
Despite their structural similarities, ortho-phenylenes and 2,3-quinoxalinylenes (i.e., poly(quinoxaline-2,3-diyl)s), well-known as foldamers and helical polymers, respectively, exhibit distinctly different conformational behavior. o-Phenylenes tend to fold into compact helices with every fourth ring stacked, whereas 2,3-quinoxalinylenes favor extended helices with no backbone stacking. To understand this difference, we have studied short o-arylenes with different sequences of benzene and pyrazine units. Through a combination of crystallography, variable-temperature NMR spectroscopy, and DFT calculations, we find that pyrazines favor extended helical conformations as a result of two effects. First, within an o-arylene architecture, pyrazines experience weaker arene–arene interactions. Cofacial packing of the rings is therefore less favorable. Second, bipyrazine units lead to an increase in vibrational entropy for extended conformers. Consequently, at higher temperatures (including room temperature), extended helices are favored for the heterocycle-containing systems.
