Viraj C. Kirinda, Briana R. Schrage, Christopher J. Ziegler, and C. Scott Hartley*
Eur. J. Org. Chem. 2020, 5620–5625
[Published version | NSF-PAR | Preprint | Raw data]
Abstract
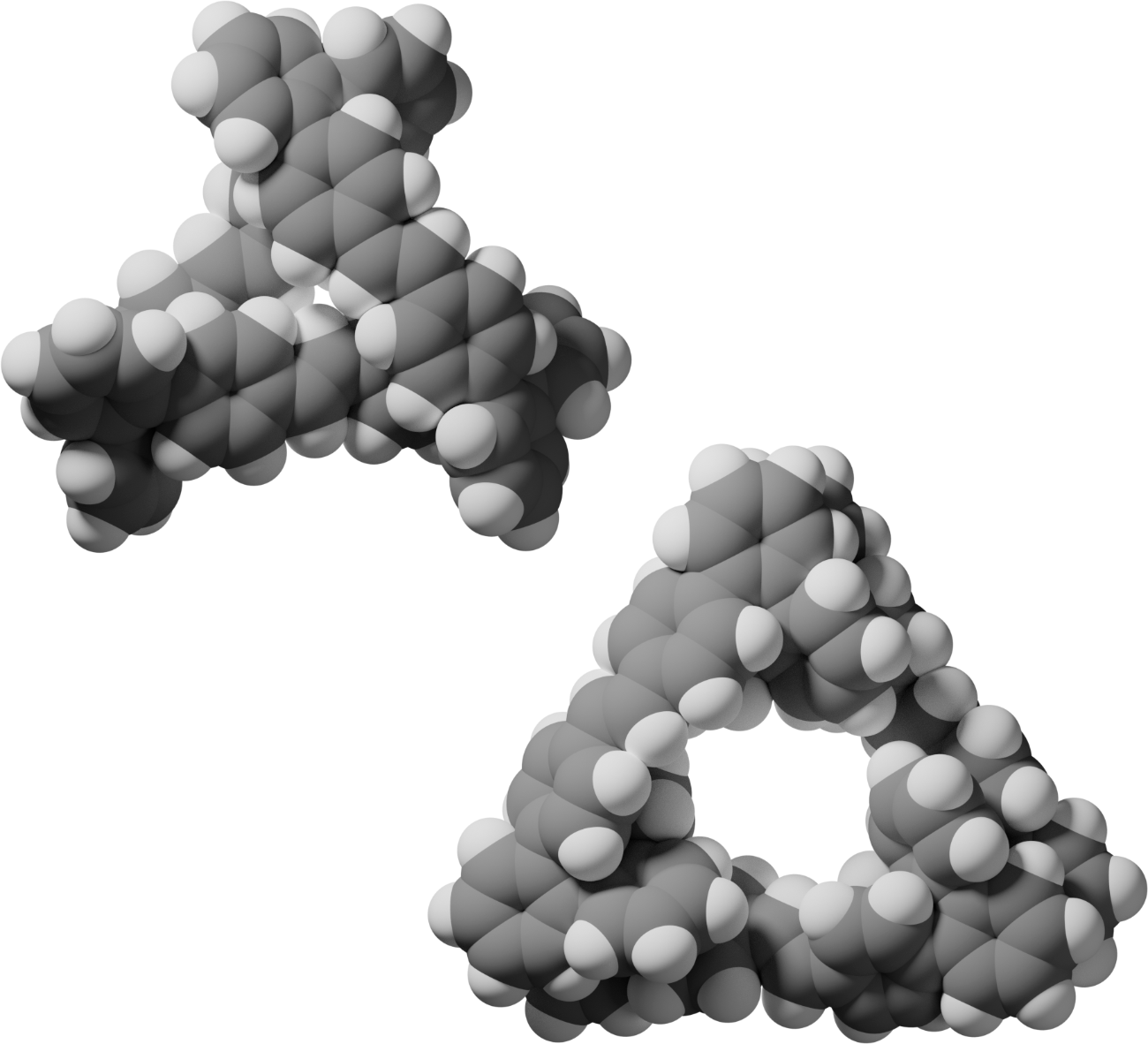
While many foldamer systems reliably fold into well‐defined secondary structures, higher order structure remains a challenge. A simple strategy for the organization of folded subunits in space is to link them together within a macrocycle. Previous work has shown that o‐phenylenes can be co‐assembled with rod‐shaped linkers into twisted macrocycles, showing an interesting synergy between folding and thermodynamically controlled macrocyclization. In these systems the foldamer units were largely decoupled from each other both conformationally and electronically. Here, we show that hydrocarbon macrocycles, with very short ethenylene linkers, can be assembled from o‐phenylenes using olefin metathesis. Characterization by NMR spectroscopy, X‐ray crystallography, and ab initio calculations shows that the products are approximately triangular trimer macrocycles with helical o‐phenylene corners in a heterochiral configuration. Their photophysics are dominated by the 4,4′‐diphenylstilbene moieties, the longest conjugated segments, with further conjugation broken by the twisting of the o‐phenylenes.
