Jian He, Jason L. Crase, Shriya H. Wadumethrige, Khushabu Thakur, Lin Dai, Shouzhong Zou, Rajendra Rathore, and C. Scott Hartley*
J. Am. Chem. Soc. 2010, 132, 13848–13857
[Published version]
Abstract
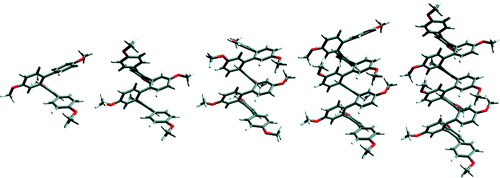
ortho-Phenylenes represent a fundamental but relatively unexplored class of conjugated molecular architecture. We have developed a robust synthetic approach to monodisperse o-phenylene oligomers which we have demonstrated by synthesizing a homologous series up to the dodecamer. The o-phenylenes exhibit complex conformational behavior but are biased toward a specific 2-fold-symmetric conformation which we believe corresponds to a stacked helix. Surprisingly, the series exhibits long-range delocalization, as measured by bathochromic shifts in UV/vis spectra. Although the overall magnitude of the shifts is modest (but comparable to some other classes of conjugated materials), the effective conjugation length of the series is approximately eight repeat units. The oligomers also exhibit an unusual hypsochromic shift in their fluorescence spectra with increasing length. The origin of these trends is discussed in the context of conformational analysis and DFT calculations of the frontier molecular orbitals for the series.
