Renuka Baral, Jackson V. Gunn, and C. Scott Hartley*
ChemSystemsChem, in press
[Published version | DOE-PAGES | Preprint | Raw data]
Abstract
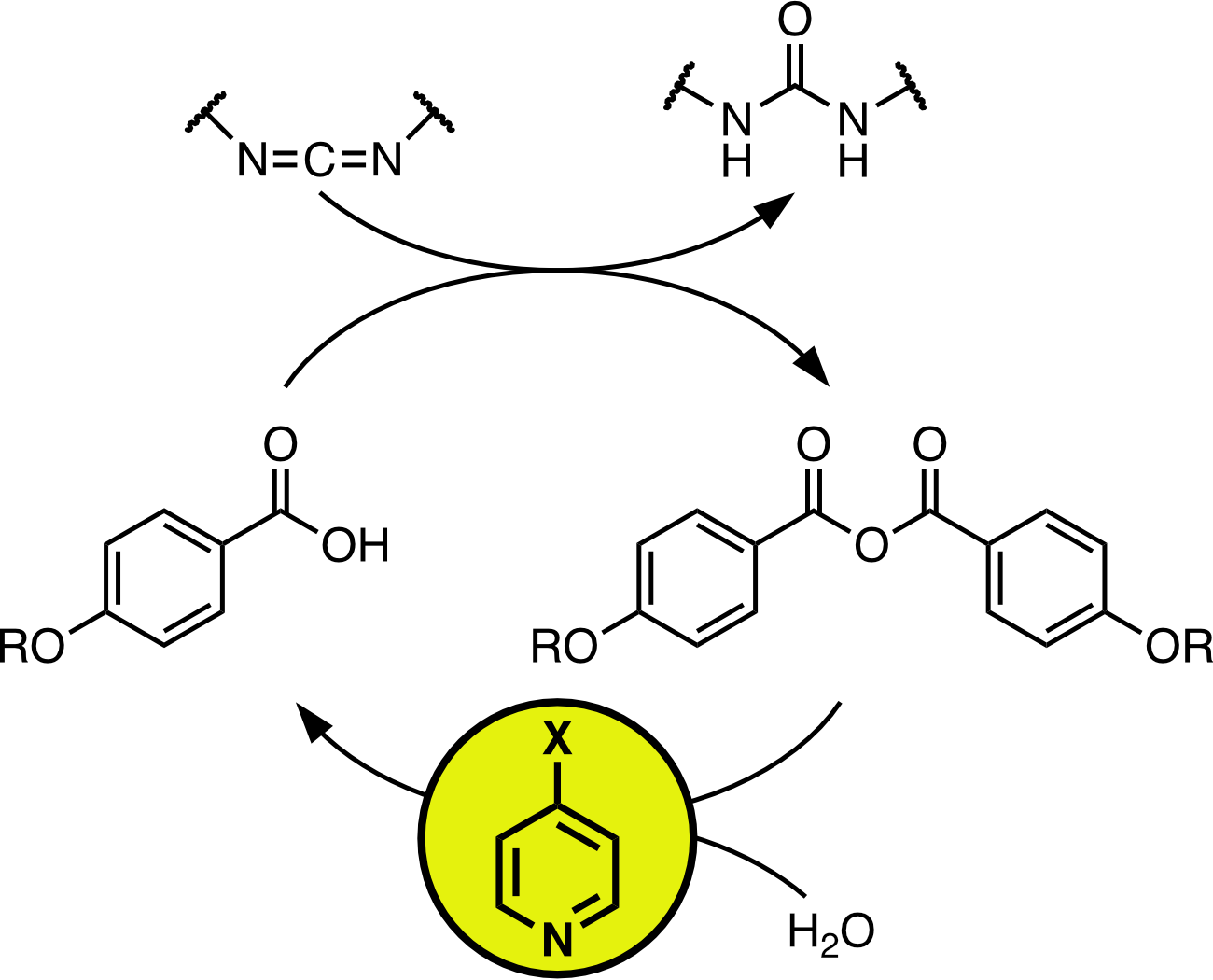
Carbodiimide-fueled reaction networks offer a versatile platform for nonequilibrium chemical systems. Typically, the carbodiimide converts a carboxylic acid to its anhydride, called “activation”, which subsequently undergoes hydrolysis, called “deactivation”. Here, we investigate pyridines with variable nucleophilicity as catalysts to control deactivation (pyridine, 4-methylpyridine, 4-methoxypyridine, and 4-dimethylaminopyridine). Reactions have been monitored by NMR spectroscopy. Although this reaction network is simple, determination of well-defined rate constants from kinetic modeling is challenging because of correlation between the parameters. This issue can be addressed by analyzing the anhydride hydrolysis independently. The rate of attack of the pyridines on the anhydride follows expected nucleophilicity trends, although this is offset by increased protonation of more-nucleophilic pyridines at typical pH’s. The optimized parameters can be used to model the full carbodiimide-driven process, although the presence of the common carbodiimide EDC has unanticipated effects on the anhydride hydrolysis rate. The results offer context for controlling carbodiimide-fueled reaction networks through the choice of suitable catalysts and pH.
