Renuka Baral, Jackson V. Gunn, and C. Scott Hartley*
J. Chem. Educ. 2025, 102, 4611–4615
[Published version | NSF-PAR | Preprint]
Abstract
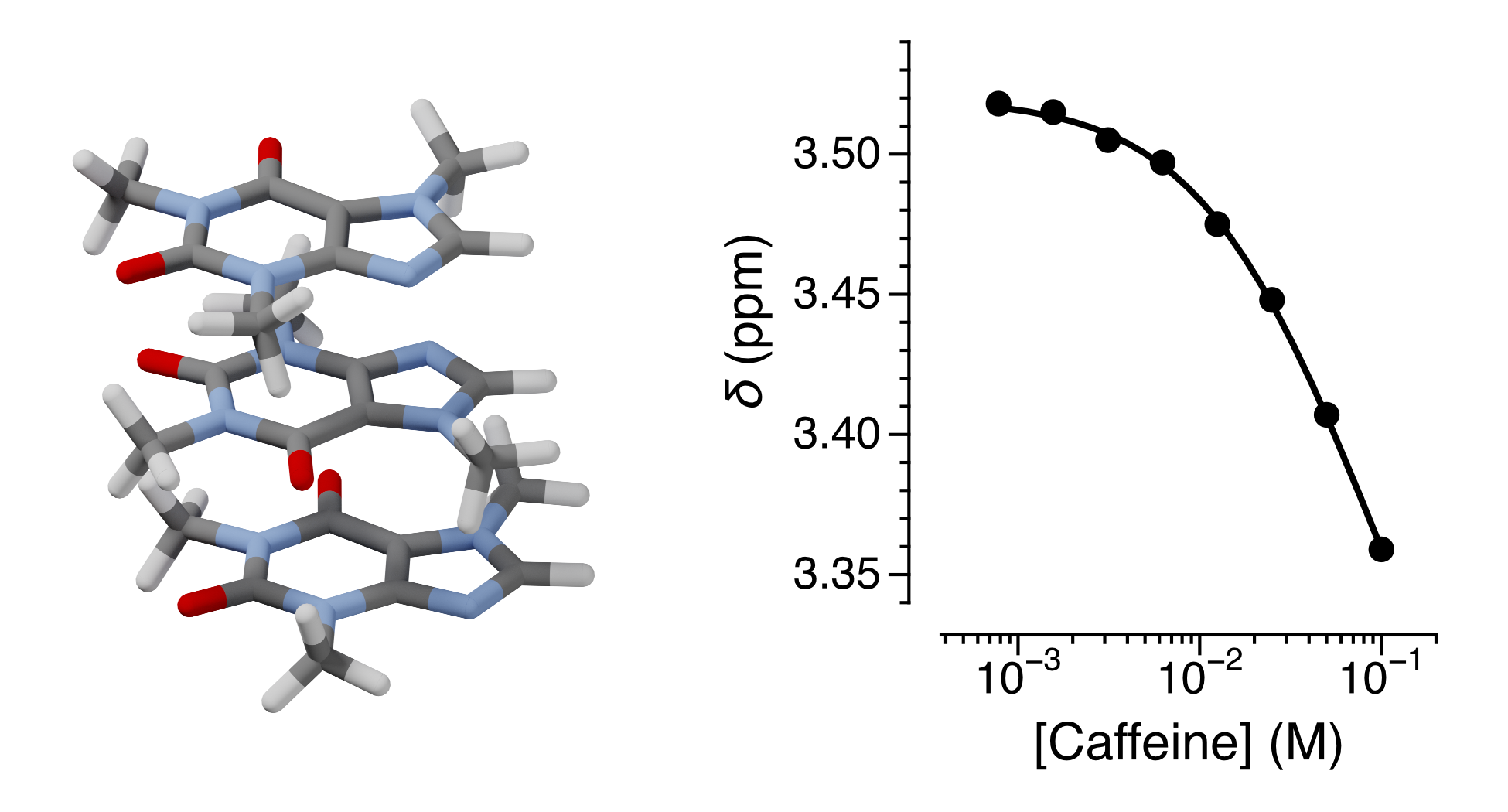
The self-association of caffeine in solution, driven by the hydrophobic effect, is a simple example of molecular aggregation. It obeys an isodesmic association model in which each successive binding occurs with the same equilibrium constant. Here, we describe an activity for chemistry students that explores this phenomenon using nuclear magnetic resonance (NMR) spectroscopy. By analyzing concentration-dependent chemical shift changes in the 1H NMR spectra of caffeine dissolved in D2O, students are introduced to uses of NMR spectroscopy beyond structure elucidation. They gain hands-on experience in quantifying supramolecular equilibria and consider their results in the context of molecular-level interactions.
