Paul J. Repasky, Deña M. Agra-Kooijman, Satyendra Kumar,* and C. Scott Hartley*
J. Phys. Chem. B 2016, 120, 2829–2837
[Published version]
Abstract
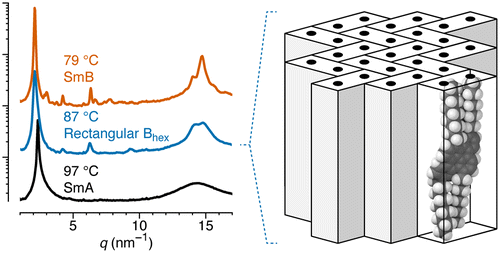
Despite longstanding interest in liquid crystalline compounds with simple rod- or disc-like shapes (calamitics or discotics), very few examples of the analogous board-shaped, or “sanidic”, liquid crystals exist. A new series of alkyl-substituted dibenzo[fg,op]naphthacenes have been prepared by planarization of o-phenylene precursors through dehydrohalogenation. Their photophysical properties have been studied in dichloromethane. Liquid crystal phase behavior was characterized by polarized optical microscopy, differential scanning calorimetry, and X-ray diffraction. All of the compounds exhibit monotropic liquid crystal phases on cooling from the isotropic phase. The compounds with shorter alkyl (pentyl and heptyl) chains exhibit the uniaxial smectic-A phase analogous to that of simple calamitic mesogens. The compounds with longer alkyl (nonyl, undecyl, and tridecyl) chains exhibit a new smectic liquid crystal phase featuring short-range positional order with an apparent rectangular lattice in the smectic layers, that is, an orthogonal biaxial hexatic-B. The molecular arrangement in this phase likely corresponds to a distorted herringbone packing of the board-shaped structures. Further, the compound with nonyl chains exhibits an underlying smectic-B phase. DFT calculations show that the cores of the mesogens are twisted into C2-symmetric saddle-shaped geometries because of steric interactions along their rims. The liquid crystal phases and their structures are discussed in the context of the compounds’ board-like shapes and intercore interactions.
