Lasith S. Kariyawasam, Julie C. Kron, Run Jiang, André J. Sommer, and C. Scott Hartley*
J. Org. Chem. 2020, 85, 682–690
[Published version | DOE-PAGES | Preprint | Raw data]
Abstract
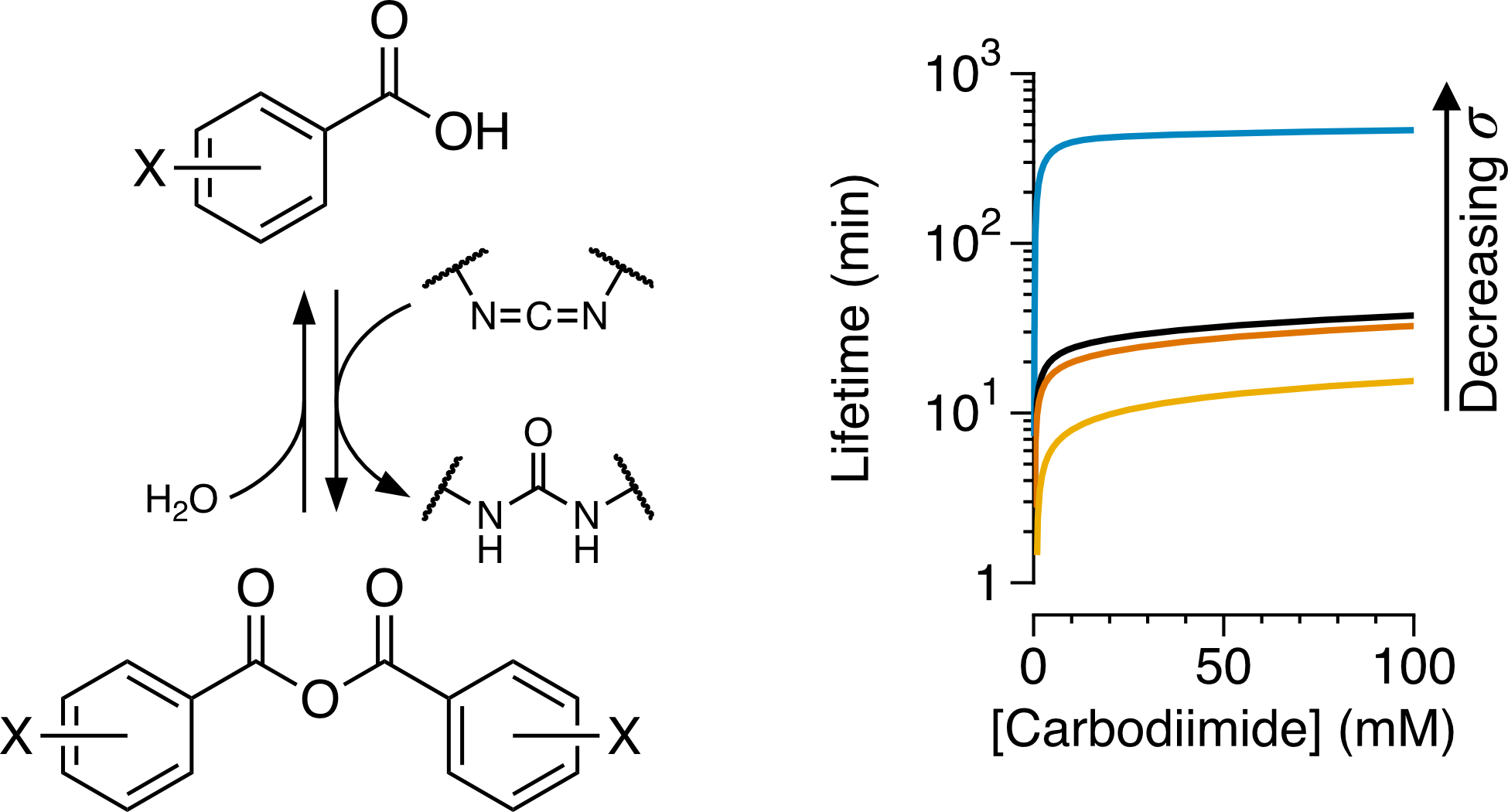
The design of dissipative systems, which operate out-of-equilibrium by consuming chemical fuels, is challenging. As yet, there are few examples of privileged fuel chemistry that can be broadly applied in abiotic systems in the same way that ATP hydrolysis is exploited throughout biochemistry. The key issue is that designing nonequilibrium systems is inherently about balancing the relative rates of coupled processes. The use of carbodiimides as fuels to generate transient aqueous carboxylic anhydrides has recently been used in examples of new nonequilibrium materials and supramolecular assemblies. Here, we explore the kinetics of formation and decomposition of a series of benzoic anhydrides generated from the corresponding acids and EDC under prototypical conditions (EDC = N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride). The reactions can be described by a simple mechanism that merges known behavior for the two processes independently. Structure–property effects in these systems are dominated by differences in anhydride decomposition rate. The kinetic parameters allow trends in concentration-dependent properties to be simulated, such as reaction lifetimes, peak anhydride concentrations, and efficiencies (i.e., total anhydride produced per equivalent of carbodiimide). For key properties there are diminishing returns with the addition of increasing amounts of fuel. This is particularly significant for the lifetimes, where substituent effects exert a much greater influence than fuel quantity under typical conditions. These results should provide useful guidelines for the design of functional systems making use of this chemistry.
