Isuru M. Jayalath, Madelyn M. Gerken, Georgia Mantel, and C. Scott Hartley*
J. Org. Chem. 2021, 86, 12024–12033
[Published version | DOE-PAGES | Preprint | Raw data]
Abstract
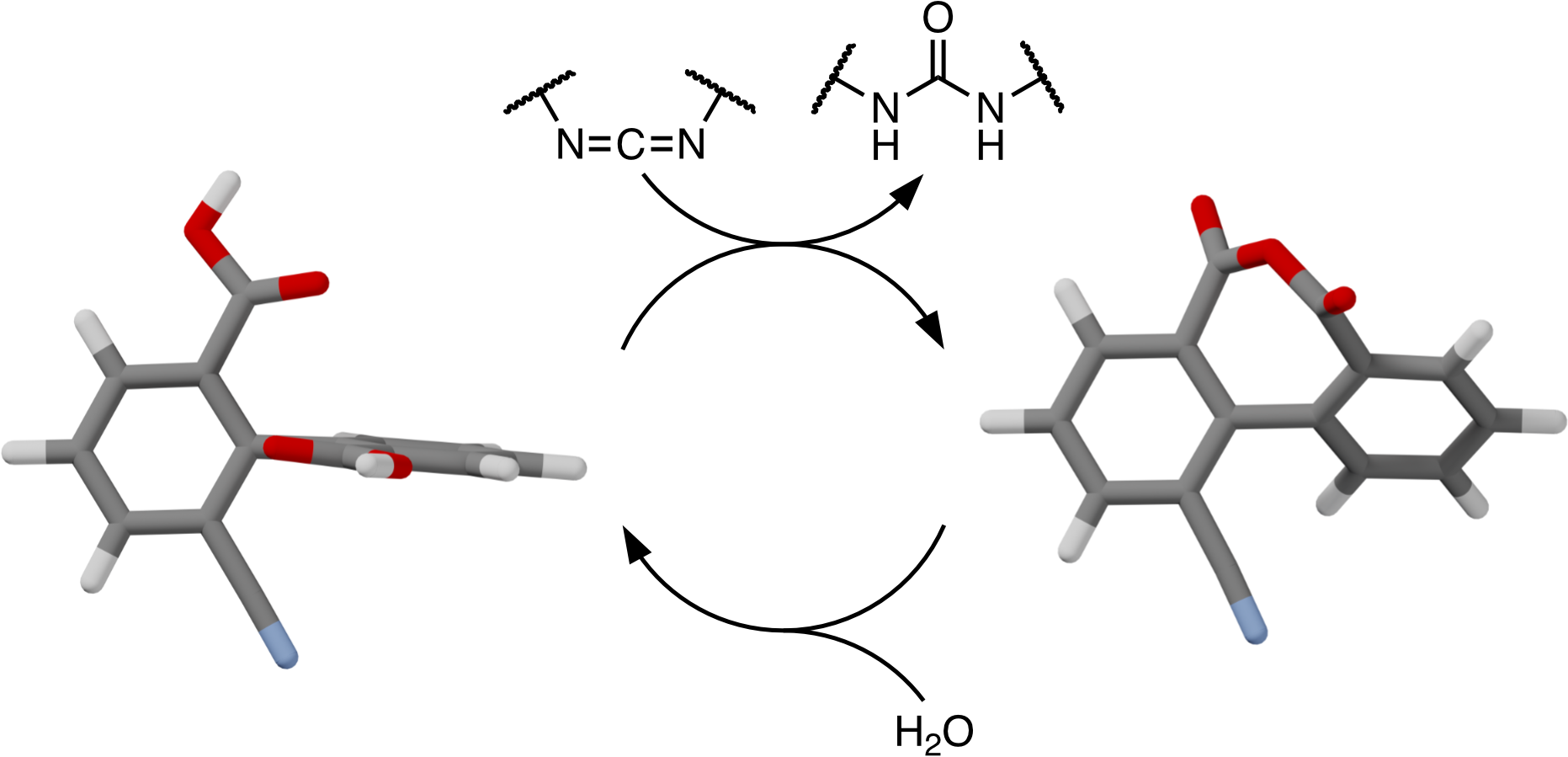
Nucleotide-induced conformational changes in motor proteins are key to many important cell functions. Inspired by this biological behavior, we report a simple chemically fueled system that exhibits carbodiimide-induced geometry changes. Bridging via transient anhydride formation leads to a significant reduction of the twist about the biaryl bond of substituted diphenic acids, giving a simple molecular clamp. The kinetics are well-described by a simple mechanism, allowing structure–property effects to be determined. The kinetic parameters can be used to derive important characteristics of the system such as the efficiencies (anhydride yields), maximum anhydride concentrations, and overall lifetimes. Transient diphenic anhydrides tolerate steric hindrance ortho to the biaryl bond but are significantly affected by electronic effects, with electron-deficient substituents giving lower yields, peak conversions, and lifetimes. The results provide useful guidelines for the design of functional systems incorporating diphenic acid units.
