Gopi Nath Vemuri, Rathiesh R. Pandian, Brian J. Spinello, Erika B. Stopler, Zacharias J. Kinney, and C. Scott Hartley*
Chem. Sci. 2018, 9, 8260–8270
[Published version | NSF-PAR | Raw data]
Abstract
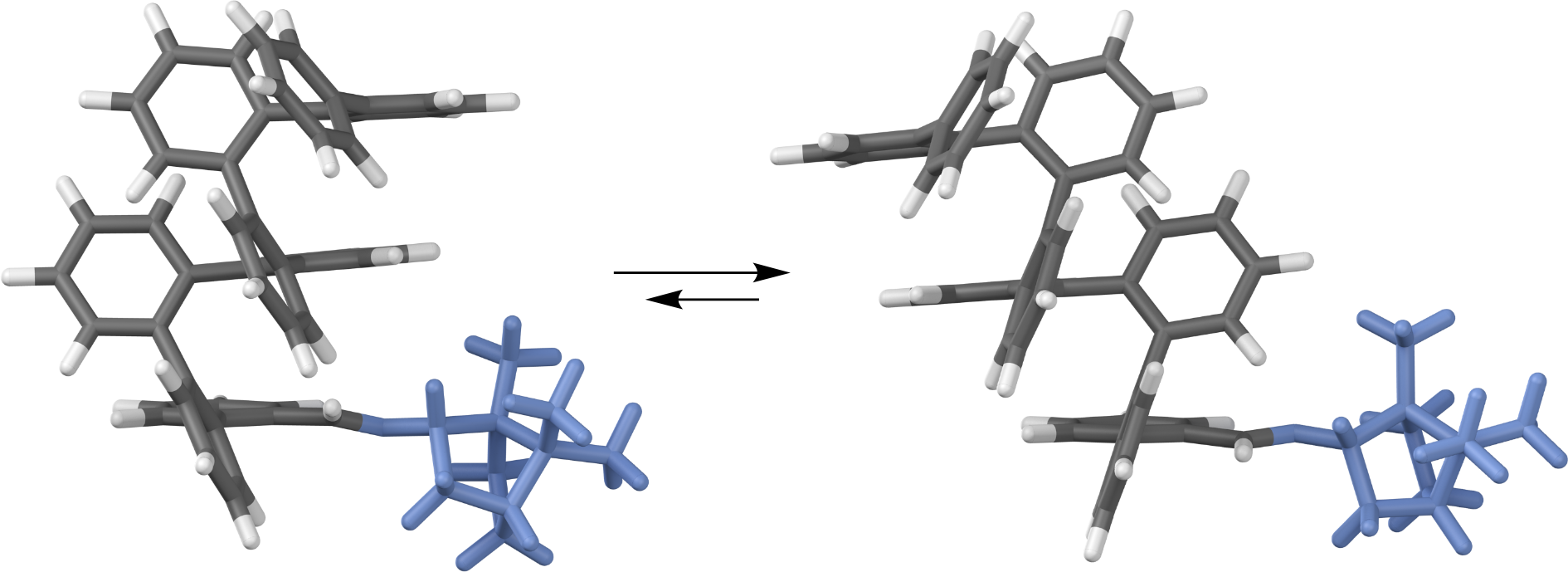
Many abiotic foldamers are based on achiral repeat units but adopt chiral geometries, especially helices. In these systems, there is no inherent preference for one handedness of the fold; however, it is well-established that the point chirality of substituents can be communicated to the helix. This capability represents a basic level of control over folding that is necessary for applications in molecular recognition and in the assembly of higher-order structures. The ortho-phenylenes represent a structurally simple class of aromatic foldamers that fold into helices driven by arene–arene stacking interactions. Although their folding is now reasonably well-understood, access to o-phenylenes enriched in one twist sense has been limited to resolution, yielding conformationally dynamic samples that racemize over the course of minutes to hours. Here, we report a detailed structure–property study of chiral induction from o-phenylene termini using a combination of NMR spectroscopy, CD spectroscopy, and computational chemistry. We uncover mechanistic details of chiral induction and show that the same substituents can give effective twist sense control in opposite directions in mixtures of interconverting conformers; that is, they are “ambidextrous”. This behavior should be general and can be rationalized using a simple model based on sterics, noting that arene–arene stacking is, to a first approximation, unaffected by flipping either partner. We demonstrate control over this mechanism by showing that chiral groups can be chosen such that they both favor one orientation and provide effective chiral induction.
