Sanyo Mathew, Laura A. Crandall, Christopher J. Ziegler, and C. Scott Hartley*
J. Am. Chem. Soc. 2014, 136, 16666–16675
[Published version]
Abstract
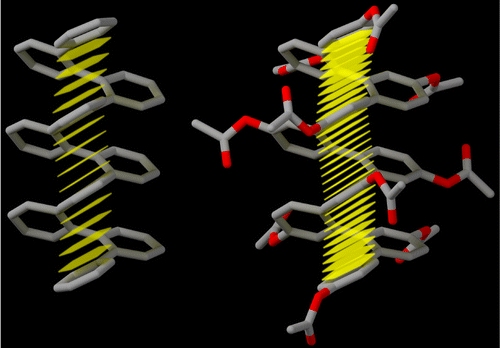
The ortho-phenylenes are a simple class of foldamers, with the formation of helices driven by offset aromatic stacking interactions parallel to the helical axis. For the majority of reported o-phenylene oligomers, the perfectly folded conformer comprises perhaps 50–75% of the total population. Given the hundreds or thousands of possible conformers for even short oligomers, this distribution represents a substantial bias toward the folded state. However, “next-generation” o-phenylenes with better folding properties are needed if these structures are to be exploited as functional units within more complex architectures. Here, we report several new series of o-phenylene oligomers, varying both the nature and orientation of the substituents on every repeat unit. The conformational behavior was probed using a combination of NMR spectroscopy, DFT calculations, and X-ray crystallography. We find that increasing the electron-withdrawing character of the substituents gives oligomers with substantially improved folding properties. With moderately electron-withdrawing groups (acetoxy), we observe >90% of the perfectly folded conformer, and stronger electron withdrawing groups (triflate, cyano) give oligomers for which misfolded states are undetectable by NMR. The folding of these oligomers is only weakly solvent-dependent. General guidelines for the assessment of o-phenylene folding by NMR and UV–vis spectroscopy are also discussed.
